filter
-
Brand
- By Category
- Direction
- Date Range
380Events
Pictures
Events

Editorial Photo illustration in Ukraine - September 30, 2024
- 2024-10-01
- 1

Editorial House Republicans slammed for not supporting women?s right to contraception bill, Washington, District of Columbia, USA - 26 Sep 2024
- 2024-09-27
- 2

Editorial BIZ-FDA-ABBVIE-TB
- 2024-09-13
- 1

Editorial FL: FDA wants to Lower Sodium Consumption in Packaged and Processed Foods by about 20%
- 2024-08-18
- 12

Editorial Human Receives A Titanium Heart For The First Time In Medical History
- 2024-08-05
- 17
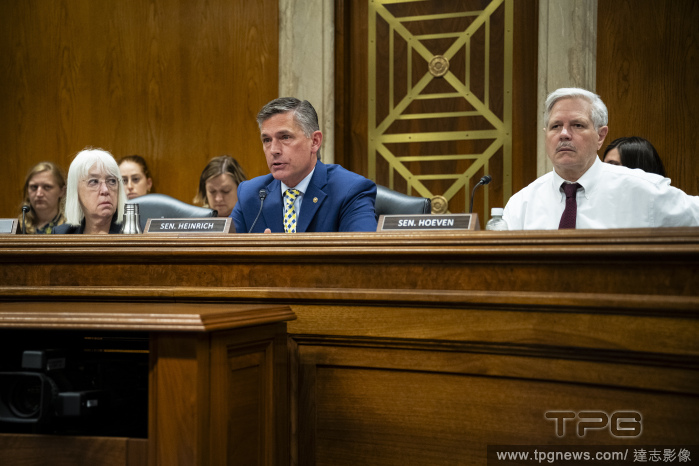
Editorial DC: U.S. Congress, Senate Appropriations Subcommittee Hearing on FDA Budget
- 2024-05-09
- 15

Editorial FDA Commissioner Robert Califf at a Congressional Hearing
- 2024-04-12
- 4

Editorial FDA Commissioner Dr. Robert Califf
- 2024-04-12
- 3

Editorial Protests at the Supreme Court as it hears case for abortion pill access, Washington, United States - 26 Mar 2024
- 2024-03-27
- 4

Editorial Protests at the Supreme Court as it hears case for abortion pill access, Washington, United States - 26 Mar 2024
- 2024-03-26
- 1

Editorial US-NEWS-FDA-CINNAMON-LEAD-WARNING-MI
- 2024-03-07
- 1

Editorial Symposium on AI use in medical sector
- 2024-02-26
- 1

Editorial Senator Schumer Urges FTC
- 2024-01-23
- 12

Editorial Senator Schumer urges FTC and FDA to Investigate 'ZYN', New York, USA - 21 Jan 2024
- 2024-01-22
- 12

Editorial Senator Schumer Urges FTC & FDA To Investigate ''ZYN'' For Marketing & Health Concerns Related To Kids
- 2024-01-22
- 12

Editorial Senator Schumer Urges FTC & FDA To Investigate "ZYN" For Marketing & Health Concerns Related To Kids - 21 Jan 2024
- 2024-01-22
- 12

Editorial NY: CVS to pull phenylephrine products from shelves
- 2023-10-21
- 3

Editorial CVS to pull phenylephrine products from shelves
- 2023-10-21
- 3

Editorial President Biden and Vice President Harris Deliver Remarks on Lowering Health Care Costs, Washington, DC, USA - 29 Aug 2023
- 2023-08-30
- 2

Editorial Prime energy drinks
- 2023-07-13
- 1

Editorial Prime energy drinks
- 2023-07-13
- 2

Editorial Prime energy drinks
- 2023-07-13
- 2

Editorial NY: Prime energy drinks
- 2023-07-12
- 5

Editorial Senator Schumer Calls on the FDA to investigate PRIME in New York - 09 Jul 2023
- 2023-07-12
- 20

Editorial Senator Schumer Calls on the FDA to investigate PRIME
- 2023-07-11
- 20

Editorial Senator Schumer Calls on the FDA to investigate PRIME, New York, USA - 09 Jul 2023
- 2023-07-10
- 40

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-27
- 2

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-25
- 8

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-20
- 8

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-20
- 8

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-20
- 10

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-20
- 9

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-20
- 8

Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-06-19
- 7
Editorial US-NEWS-KY-OPIOIDS-PSYCHEDELICS-2-LX
- 2023-05-20
- 4

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-05-16
- 1

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-05-11
- 1

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-05-11
- 1

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-05-05
- 21

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-22
- 3

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-22
- 1

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-20
- 2

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-20
- 3

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-19
- 1

Editorial The Food and Drug Administration building in Silver Spring, Md., on May 26, 2022. (Sarahbeth Man/The New York Times)
- 2023-04-16
- 12
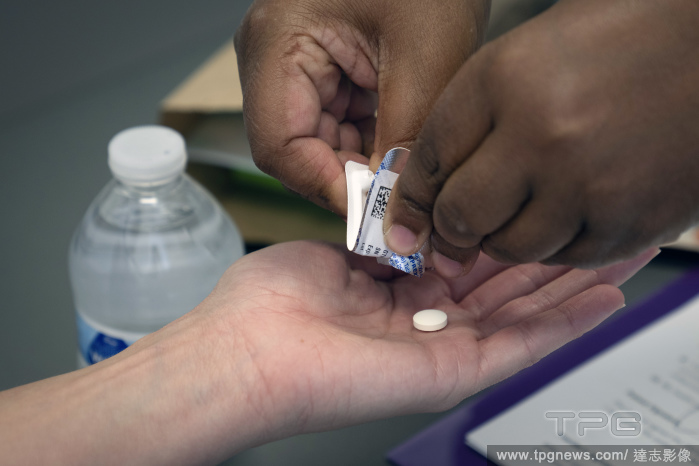
Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-04-12
- 1

Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-03-29
- 5

Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-03-22
- 8

Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-03-21
- 7

Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-03-20
- 7

Editorial A nurse midwife dispenses a dose of mifepristone at Choices Center for Reproductive Health in Carbondale, Ill., on Oct. 25, 2022. (Erin Schaff/The New York Times)
- 2023-03-20
- 6
Editorial US-NEWS-CORONAVIRUS-FDA-DRUG-TB
- 2023-01-28
- 1

Editorial NY: FDA wants simpler COVID vaccine process
- 2023-01-24
- 5

Editorial FDA logo, Motala, Sweden - 07 Jan 2023
- 2023-01-08
- 7

Editorial LIFE-HEALTH-FLA-KETAMINE-1-OS
- 2022-11-03
- 1

Editorial Hearing Aids
- 2022-10-21
- 4

Editorial Dog Food Heart Disease, Toronto, Canada - 07 Jul 2019
- 2022-10-20
- 1

Editorial From left: Dr. Rochelle Walensky, the CDC director, Dr. Anthony Fauci, the Biden administration?s top medical adviser, and Dr. Robert Califf, the FDA commissioner, during a Senate hearing on monkeypox, on Capitol Hill in Washington on Sept. 14, 2022. (Anna Rose Layden/The New York Times)
- 2022-09-29
- 1

Editorial DC: U.S. Capitol, Senate Health Committee Hearing on Monkeypox
- 2022-09-16
- 5

Editorial US-NEWS-ALS-DRUG-FL
- 2022-09-15
- 1

Editorial A health care worker prepares a COVID-19 vaccine in Jackson, Ala., Oct. 5, 2021. (Charity Rachelle/The New York Times)
- 2022-09-01
- 1

Editorial Three batches of Banana Boat Hair & Scalp Sunscreen were recalled because they contained trace levels of benzene, a human carcinogen. (via U.S. Food and Drug Administration via The New York Times)
- 2022-08-01
- 1

Editorial US-NEWS-CORONAVIRUS-FAKE-TREATMENTS-CH
- 2022-07-29
- 1

Editorial FDA Commissioner Robert Califf at a Senate Hearing
- 2022-07-22
- 3

Editorial In a photo provided by the FDA shows an undated image of an ultraviolet wand. (U.S. Food and Drug Administration via The New York Times)
- 2022-07-21
- 1

Editorial FDA to fly into Haneda for the first time
- 2022-07-12
- 7

Editorial Birth control pills in Philadelphia, Nov. 16, 2021. (Hannah Yoon/The New York Times)
- 2022-07-12
- 2

Editorial A woman receives a COVID-19 vaccine booster shot in San Juan, P.R., on May 20, 2022. (Erika P. Rodriguez/The New York Times)
- 2022-07-04
- 1

Editorial A vaccine site offering services to people over the age of five in Los Angeles, March 31, 2022. (Alisha Jucevic/The New York Times)
- 2022-06-16
- 1

Editorial US-NEWS-MD-CANNABIS-DELTA8-2-BZ
- 2022-05-23
- 1

Editorial NY: FDA Proposes Ban On Menthol Cigarettes
- 2022-05-18
- 7

Editorial NY: 20th Anniversary of Botox in New York
- 2022-04-27
- 4

Editorial 20th Anniversary of Botox in New York
- 2022-04-27
- 4

Editorial FDA investigating Lucky Charms illnesses
- 2022-04-19
- 3

Editorial NY: FDA investigating Lucky Charms illnesses
- 2022-04-19
- 3

Editorial Dr. Washington Hill, a maternal-fetal medicine specialist, in Sarasota, Fla.,on Feb. 14, 2022. (Octavio Jones/The New York Times)
- 2022-03-29
- 1

Editorial Dr. Washington Hill, a maternal-fetal medicine specialist, in Sarasota, Fla.,on Feb. 14, 2022. (Octavio Jones/The New York Times)
- 2022-03-26
- 1

Editorial An undated photo provided by the Food and Drug Administration shows FDA field inspectors checking imported seafood at the Los Angeles International Airport in 2009. (Food and Drug Administration via The New York Times)
- 2022-03-22
- 1

Editorial Lizzie Burgess in Fishers, Ind., March 3, 2022. (Lee Klafczynski/The New York Times)
- 2022-03-09
- 1

Editorial BIZ-FDA-ABBOTT-TB
- 2022-03-03
- 2

Editorial US-NEWS-MED-FDA-CONDOMS-LX
- 2022-02-24
- 1

Editorial US-NEWS-MED-FDA-CONDOMS-LX
- 2022-02-22
- 28

Editorial Senators After Confirming FDA Commissioner, Washington, District of Columbia, United States - 15 Feb 2022
- 2022-02-18
- 21

Editorial United States Senator Elizabeth Warren, a Democrat from Massachusetts, walks to the Senate to vote on Robert McKinnon Califf to be Commissioner of the Food and Drug Administration (FDA) at the US Capitol in Washington, DC on Tuesday, February 15, 2022.
- 2022-02-16
- 28

Editorial DC: Senate votes on Robert McKinnon Califf to be Commissioner of the Food and Drug Administration
- 2022-02-16
- 28

Editorial Thailand pushes to legalize recreational marijuana in Nakhon Ratchasima - 26 Jan 2022
- 2022-01-26
- 13

Editorial US-NEWS-CORONAVIRUS-OMICRON-THERAPIES-FL
- 2022-01-25
- 1

Editorial NY: FDA frees French Dressing
- 2022-01-17
- 2

Editorial FDA frees French Dressing
- 2022-01-17
- 1

Editorial FDA frees French Dressing
- 2022-01-17
- 1

Editorial Senate Health, Education Committee Hearing on COVID-19, Washington, District of Columbia, United States - 11 Jan 2022
- 2022-01-12
- 1

Editorial Protective Face Masks Photo Illustrations, Krakow, Poland - 11 Jan 2022
- 2022-01-12
- 1

Editorial Senate Health, Education Committee Hearing on COVID-19
- 2022-01-12
- 1

Editorial CES 2022, Media Days, Day1, Las Vegas, United States - 03 Jan 2022
- 2022-01-04
- 2

Editorial Regina Paramo, 12, receives a dose of a vaccine in San Antonio, Texas on May 13, 2021. The FDA will allow Pfizer boosters for 12- to 15-year-olds, say those familiar with the plan. (Tamir Kalifa for The New York Times)
- 2021-12-31
- 1

Editorial FDA authorizes Merck's COVID-19 anti-viral pill
- 2021-12-24
- 5

Editorial Robert Califf at a Senate Health, Education, Labor, and Pensions Committee Hearing
- 2021-12-15
- 6

Editorial DC: U.S. Capitol, Senate HELP Committee Nomination Hearing for FDA Commissioner
- 2021-12-15
- 32

Editorial Samantha Miller, co-cief executive of Cadence Health, in Piedmont, Calif., on Oct. 10, 2021. (Carolyn Fong/The New York Times)
- 2021-12-14
- 1

Editorial Photo illustration in Ukraine - 09 Dec 2021
- 2021-12-10
- 1
 Loading
Loading 