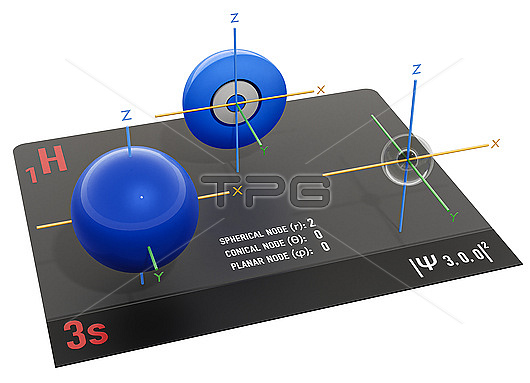
3s electron orbital, illustration. An electron orbital is a region surrounding an atomic nucleus (not visible) where one or a pair of electrons is most likely to exist. The orbital is seen halved at middle to show the axes of symmetry and the concentric spherical nodes can be seen at right. Nodes are the regions in an atom with zero electron density and where the electron is least likely to exist. For the 3s electron orbital, 3 indicates that it is the third energy level, and s indicates that it is a spherical and symmetrical orbital. The 3s orbital can accommodate up to 2 electrons. It is larger and more diffuse compared to the 1s and 2s orbitals because it is located farther from the nucleus. The 3 shell also contains three 3p orbitals and five 3d orbitals at higher energy levels.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP29775952
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
 Loading
Loading