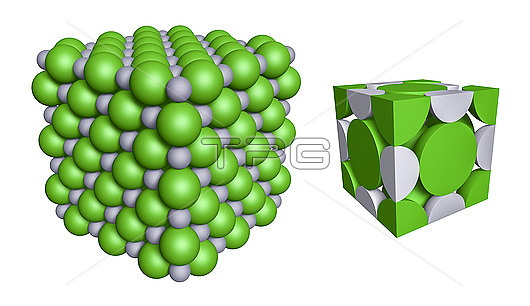
Crystal structure of sodium chloride, illustration. Sodium chloride (NaCl) crystallizes in cubic structure (left), with alternating rows of sodium cations (Na+, grey) and chloride anions (Cl?? green). The unit cell (right) has one-fourth of a sodium ion along each edge of the cube. The lattice also has one full chloride ion at the centre of the cell, one-eighth at each of the corners, and one-half a chloride ion in each face. Combined, each unit cell contains three units of NaCl.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP27695795
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading