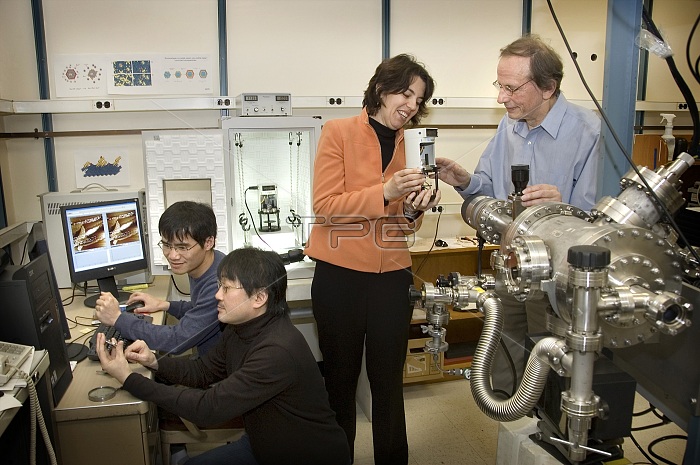
As part of an ongoing effort to produce efficient and affordable fuel cells, scientists at the DOE's Brookhaven National Laboratory are looking for ways to use gold to prevent the destruction of platinum in the chemical reactions that take place in fuel cells. Platinum is the most efficient electrocatalyst for accelerating chemical reactions in fuel cells. However, in reactions during the stop-and-go driving of a fuel-cell-powered electric car, the platinum dissolves. In accelerated tests, as much as 45 percent of the catalyst can be lost during five days. So BNL scientists are looking for a way to preserve it. A team of BNL researchers added gold clusters to a platinum electrocatalyst, which kept it intact during an accelerated stability test that simulates stop-and-go driving in an electric car. Using x-rays as probes at Brookhaven's National Synchrotron Light Source, a STM at Brookhaven's Center for Functional Nanomaterials, and electrochemical techniques in the laboratory, the scientists can show that less platinum is oxidized with this method. As predicted, during laboratory testing, the platinum electrocatalyst remains stable when under conditions mimicking stop-and-go driving conditions.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22288159
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
No
Property Release:
No
Right to Privacy:
No
Same folder images:
 Loading
Loading