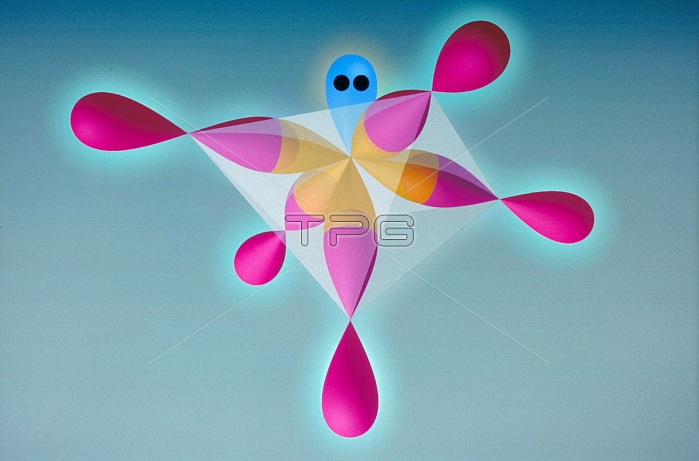
Molecular model of iodine pentafluoride (IF5), a toxic gas. The atoms are color coded: iodine (orange) and fluorine (red). The blue area is a lone pair of electrons that are not involved in a chemical bond. This molecule has a square-based pyramid shape, and is an example of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This states that bonded atoms around a central atom adopt a configuration in order to be as far away from each other as possible. Without a lone pair, atoms surrounded by five others have a trigonal bipyramidal shape.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22279708
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
No
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading