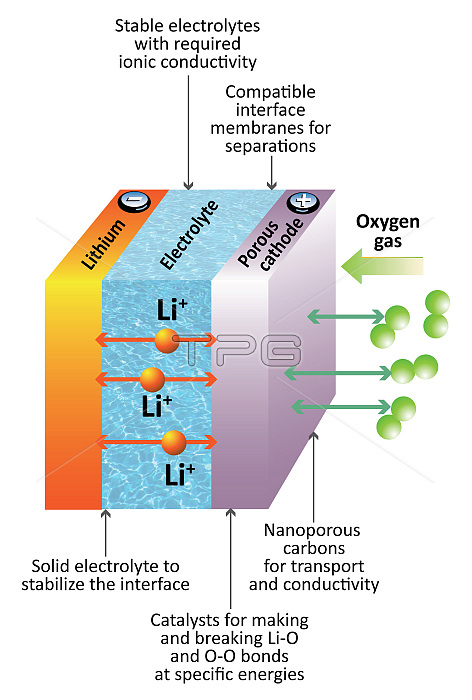
This illustration shows the inner workings of a lithium-air battery. Li-air batteries hold the promise of increasing the energy density of LI-ion batteries by as much as five to 10 times, or almost as much energy as a tank of gasoline of the same size. A Li-air battery has a positive electrode made of lightweight porous carbon and a negative electrode made of lithium metal. To make electricity, oxygen from the air moves through the porous carbon electrode, where it reacts catalytically with lithium ions and electrons from the external circuit to form a solid lithium oxide. The solid lithium oxide gradually fills the pore spaces inside the carbon electrode as the battery discharges. When the battery is recharged, the lithium oxide decomposes again, releasing lithium ions and freeing up pore space in the carbon. Resulting oxygen is released back into the atmosphere.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22089257
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading