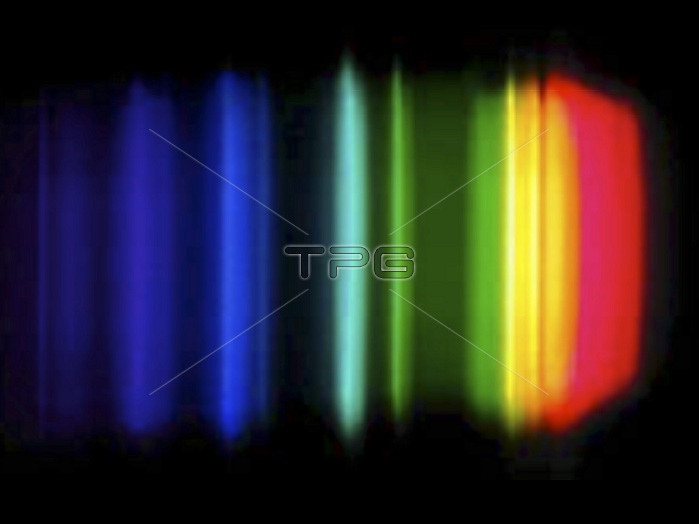
Sodium emission spectrum when reacting with water. The colours here show the electromagnetic radiation emitted during this reaction by the element sodium. Such spectra are used to identify chemical elements, both in the laboratory and in distant stars. This spectrum is dominated by the bright doublet known as sodium D-lines at 589.0 and 589.6 nanometres (nm). These lines are emitted in a transition from the 3p to the 3s atomic energy levels. The line at 589.0 has twice the intensity of the line at 589.6 nm. The strongest visible line other than the D-lines is the line at 568.8 nm. For this spectrum with a nanometre axis, see image C028/6300.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP16424940
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
 Loading
Loading