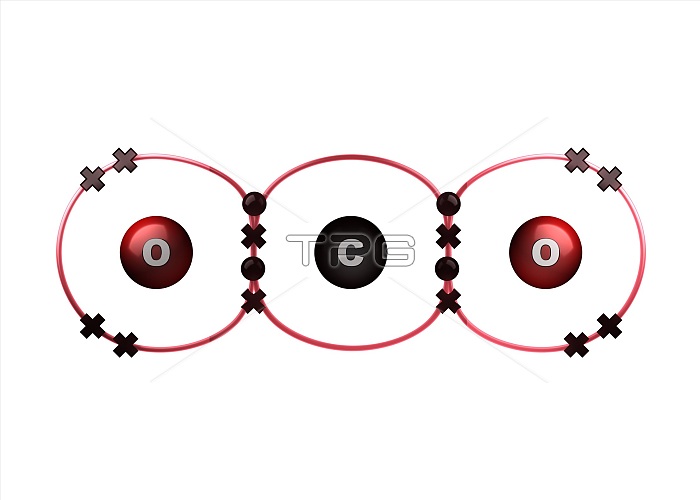
Bond formation in carbon dioxide molecule. Illustration of the sharing of electrons (dots and crosses) between two oxygen (O) and one carbon (C) atom to form a molecule of carbon dioxide (CO2). This is an example of covalent bonding, with the two double bonds each formed by two shared electron pairs, each pair consisting of an electron from each atom. The outer electron shell of each atom is shown as a red ring. Carbon dioxide is a gas at room temperature and pressure. For the atoms before the bonds form, see image C028/6474.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP16412211
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading