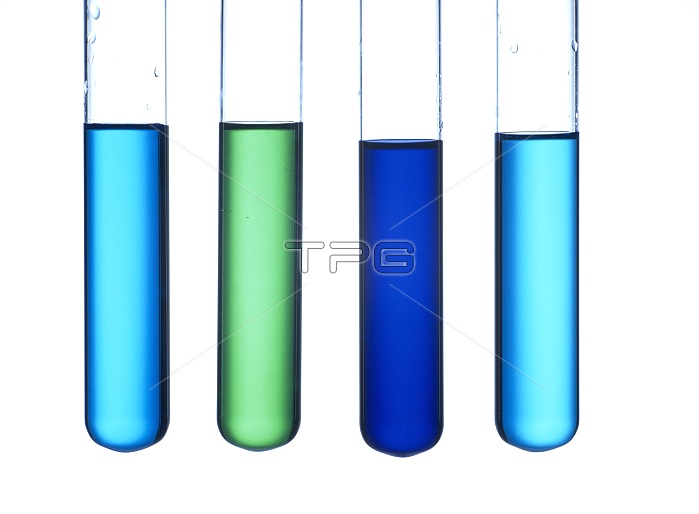
Copper ion solutions. Solution of copper II ions (Cu2-) in copper sulphate (far left). When concentrated hydrochloric acid (HCl) is added, tetrachlorocuprate II complex ions ((CuCl4)2-) are formed. These ions are yellow in colour, which with the blue colour of copper ions results in a vivid green coloured solution (centre left). On addition of ammonia (NH3), tetraaminediaqua copper II complex ions ((Cu(NH3)4(H2O))2+) are formed, which are dark blue (centre right). On addition of EDTA (ethylene-diaminetetraacetic acid), copper- EDTA complex ions (Cu(EDTA)2-) are formed, which are pale blue (far right).
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10163895
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading