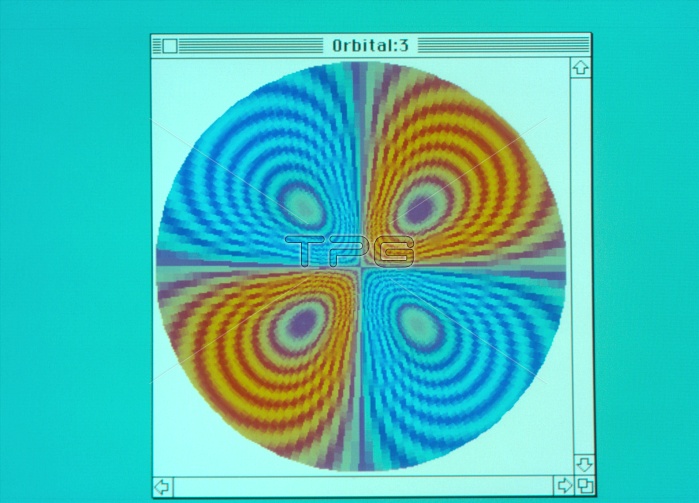
Atomic orbitals. Computer display of an electronic orbital of the hydrogen atom. The orbital shown here is known as 3d and it is formed by four symmetric lobes, two blue and two red-orange green, seen sliced in the cartesian plane xy, with the nucleus at the centre of the frame. According to the atomic quantum theory it is not possible to specify a trajectory for the electrons moving around the nucleus. The quantum theory introduces instead the concept of orbitals as regions where there is a given probability of finding the electrons. The coloured bands in the lobes show different probability levels; the probability decreases when moving away from the nucleus.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10162884
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading