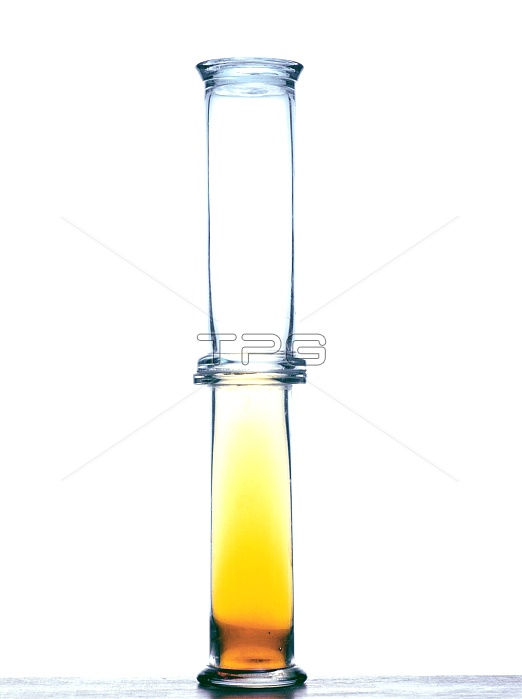
Bromine diffusion. Image 1 of 2. Bromine vapour (orange) diffusing upwards to fill two gas jars. Bromine liquid is weakly bonded, and so it will readily evaporate at room temperature. Because gas molecules can move independently of each other and do so randomly, a gas spreads out from its source in a process called diffusion. The diffusion rate depends mainly on the temperature (affecting the speed of the gas molecules) and the medium through which the diffusion takes place. In this case, the medium is air. The gas colour allows the diffusion to be observed. For the vapour diffused throughout the glass jars, see image A150/310.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10162816
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
 Loading
Loading