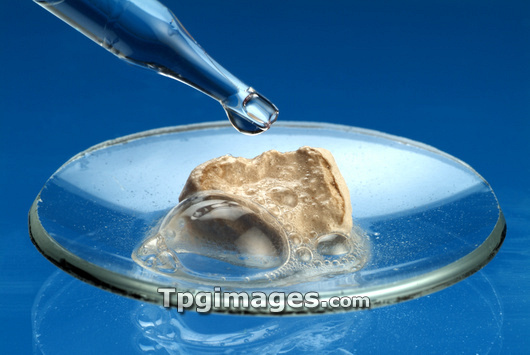
Reaction of chalk and acid. This is an example of an acid-carbonate reaction. Chalk contains calcium carbonate, and the acid here is hydrochloric acid. The reaction produces a metal salt (here calcium chloride), carbon dioxide (the bubbles seen here) and water. This reaction is the same as that which occurs when acidic rain (due to dissolved carbon dioxide producing carbonic acid) reacts with and erodes chalk.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP03197586
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading