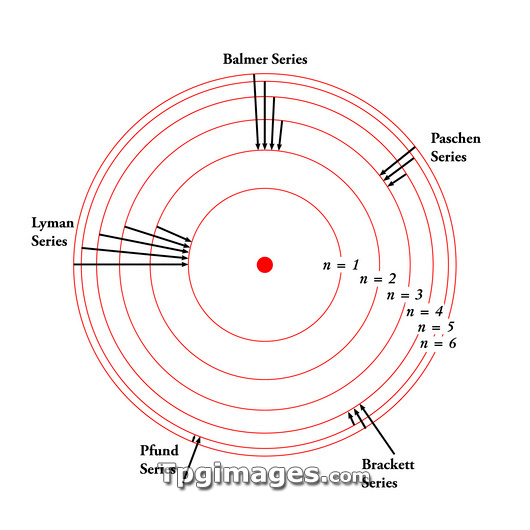
Electron transition series (black arrows) between electron orbitals (red), in the Bohr model of the hydrogen atom. Proposed in 1913 by the Danish physicist Niels Bohr (1885-1962), this model had electrons occupying discrete (quantised) energy levels (here labelled as n from 1 to 6). The orbitals surround the nucleus (red dot). When hydrogen is heated, the electrons absorb energy and rise up one or more levels. When they drop back down again, they emit photons of light, producing an emission spectrum. The series of lines on these emission spectra correspond to transitions back to the same energy levels. The series are: Lyman (n=1), Balmer (n=2), Paschen (n=3), Brackett (n=4), and Pfund (n=5).
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP03197343
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
 Loading
Loading